Medical Device Regulations Ema . the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. Medical devices are products or equipment intended for a medical purpose. Authorised representatives, importers and distributors. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical.
from archive.cancerworld.net
Medical devices are products or equipment intended for a medical purpose. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. Authorised representatives, importers and distributors. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
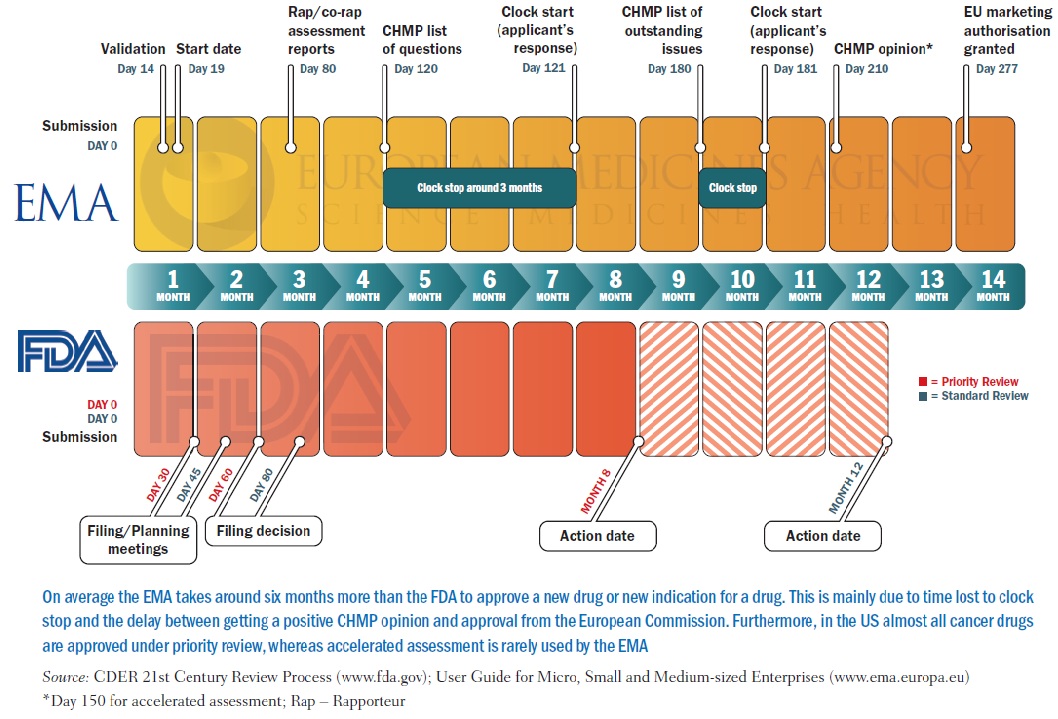
Approval rating how do the EMA and FDA compare? Cancer World Archive
Medical Device Regulations Ema regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. Medical devices are products or equipment intended for a medical purpose. Authorised representatives, importers and distributors. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical.
From www.regdesk.co
EMA Guidance on Medicines Used in Medical Devices RegDesk Medical Device Regulations Ema the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Authorised representatives, importers and distributors. Medical devices are products or equipment intended for a medical purpose. the medical devices regulation seeks to. Medical Device Regulations Ema.
From www.regdesk.co
The EMA Published Draft Guidance on DDC Products. RegDesk Medical Device Regulations Ema Authorised representatives, importers and distributors. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. Medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted. Medical Device Regulations Ema.
From www.newhivdrugs.org
Regulatory Filing Overview US FDA, WHO, GF ERP, and EMA Medical Device Regulations Ema on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. Medical devices are products or equipment intended for a medical purpose.. Medical Device Regulations Ema.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device Regulations Ema Medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. Authorised representatives, importers and distributors. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746. Medical Device Regulations Ema.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc Medical Device Regulations Ema on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. Medical devices are products or equipment intended for a medical purpose.. Medical Device Regulations Ema.
From dxoymrvmh.blob.core.windows.net
Medical Device Regulations Meaning at Sandra Shields blog Medical Device Regulations Ema regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. Authorised representatives, importers and distributors. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Medical Device Regulations Ema.
From www.presentationeze.com
Global Medical Device Regulation PresentationEZE Medical Device Regulations Ema a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. the medical. Medical Device Regulations Ema.
From www.med-technews.com
The impact of new European Medical Device Regulations MedTech Innovation Medical Device Regulations Ema Medical devices are products or equipment intended for a medical purpose. Authorised representatives, importers and distributors. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. regulation (eu). Medical Device Regulations Ema.
From www.eclevarmedtech.com
A Guide to Medical Devices Regulations Everything You Need to Know Medical Device Regulations Ema regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. Medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes. Medical Device Regulations Ema.
From alirahealth.com
Medical Device Regulation (MDR) Support Alira Health Medical Device Regulations Ema the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. Authorised representatives, importers and distributors. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. regulation (eu) 2017/745. Medical Device Regulations Ema.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc Medical Device Regulations Ema Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and. Medical Device Regulations Ema.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc Medical Device Regulations Ema the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. Medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of. Medical Device Regulations Ema.
From www.youtube.com
Regulatory Standards & Risk Management in Medical Devices YouTube Medical Device Regulations Ema Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. a new revision of the guidance available to applicants,. Medical Device Regulations Ema.
From mavink.com
Fda Medical Device Classification Chart Medical Device Regulations Ema on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. the medical device regulation (mdr), which was adopted in april. Medical Device Regulations Ema.
From www.youtube.com
EMA webinar on Article 117 of the Medical Devices Regulation EU 2017/ Medical Device Regulations Ema regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. the medical devices regulation seeks to ensure. Medical Device Regulations Ema.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Regulations Ema regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. the medical devices regulation seeks to ensure. Medical Device Regulations Ema.
From www.biomapas.com
EMA & FDA What Are the Similarities & Differences in Risk Management Medical Device Regulations Ema regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. on medical devices, amending directive 2001/83/ec, regulation (ec) no 178/2002 and regulation (ec) no. the medical devices regulation seeks to ensure. Medical Device Regulations Ema.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc Medical Device Regulations Ema Authorised representatives, importers and distributors. Medical devices are products or equipment intended for a medical purpose. a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies. the medical devices regulation seeks to ensure a high level of public health and patient safety taking into account. on medical devices, amending directive 2001/83/ec, regulation. Medical Device Regulations Ema.